enFuse Wearable Platform
The enFuse wearable platform is designed to deliver large volumes of medications subcutaneously, in which patients receive their needed treatment in a simple injection under the skin, instead of intravenously.

Large Volume Delivery
Delivery of 5 – 25 mL with a single device
Four devices can be worn simultaneously1
Flexible Administration
Enables patient self-administration in-home or HCP administration in-clinic
Compatible with common drug product viscosities (1-100cP)
- Custom needle design
Hands-Free Design
- Small, discreet, wearable with no wiring or tubing
- Allows for patient mobility during administration
FDA Approved*, Clinic Ready in 6 – 9 Months
Flexible Administration, In-Clinic or In-Home
50 +
Patents Issued
200k +
Units Manufactured
80 +
Preclinical Evaluations Completed, All Successful
10k +
Devices clinically tested on >300 patients in 25 countries
6 +
Clinical Trials Completed
8k +
Commercial Uses
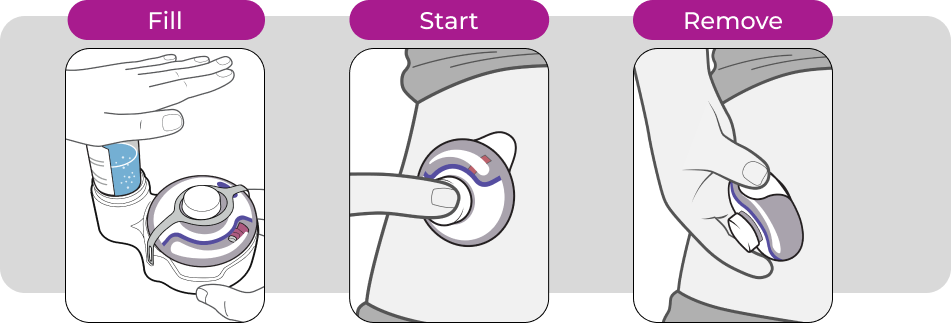
enFuse Transfer Systems
The enFuse Transfer Systems allow a user to load enFuse with the needed dose of medication from either a syringe or a vial.
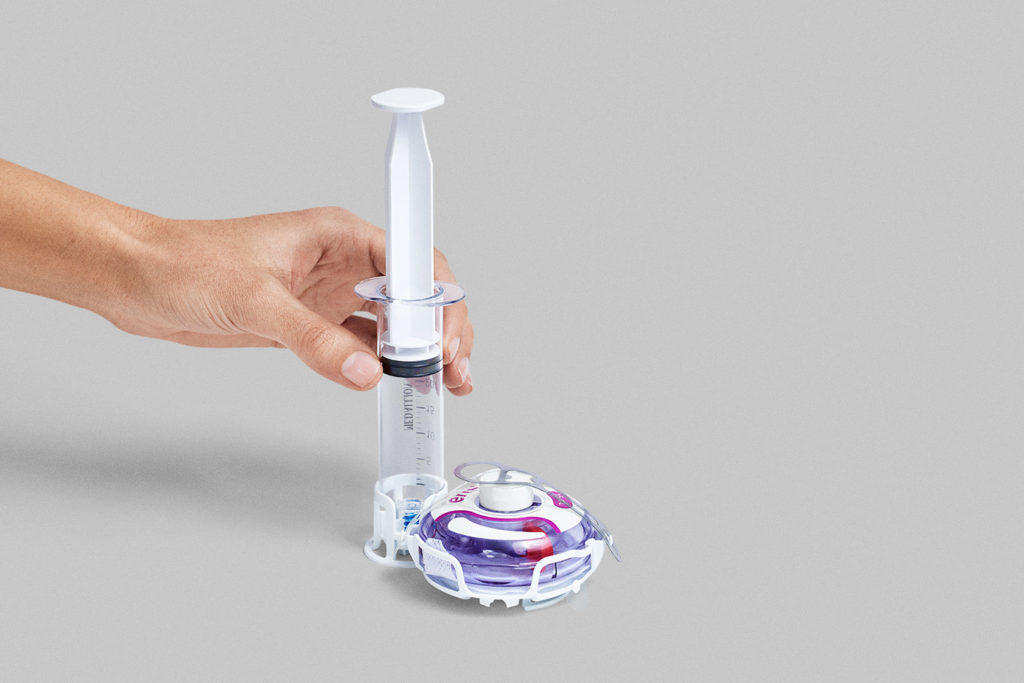
Syringe Transfer System
The enFuse Syringe Transfer System is designed for use with both pre-filled and patient-filled syringes, offering flexibility in dose volume. This system is particularly well-suited for dose-finding clinical studies where the optimal drug volume has yet to be determined.
-
Transfers drug from pre-filled or patient-filled syringes
-
Provides flexibility in dose volume transferred and administered
-
Accommodates variable and weight based dosing regimens
-
Supports early-stage clinical studies
Clinical Development Efficiency
Through Variable Dosing

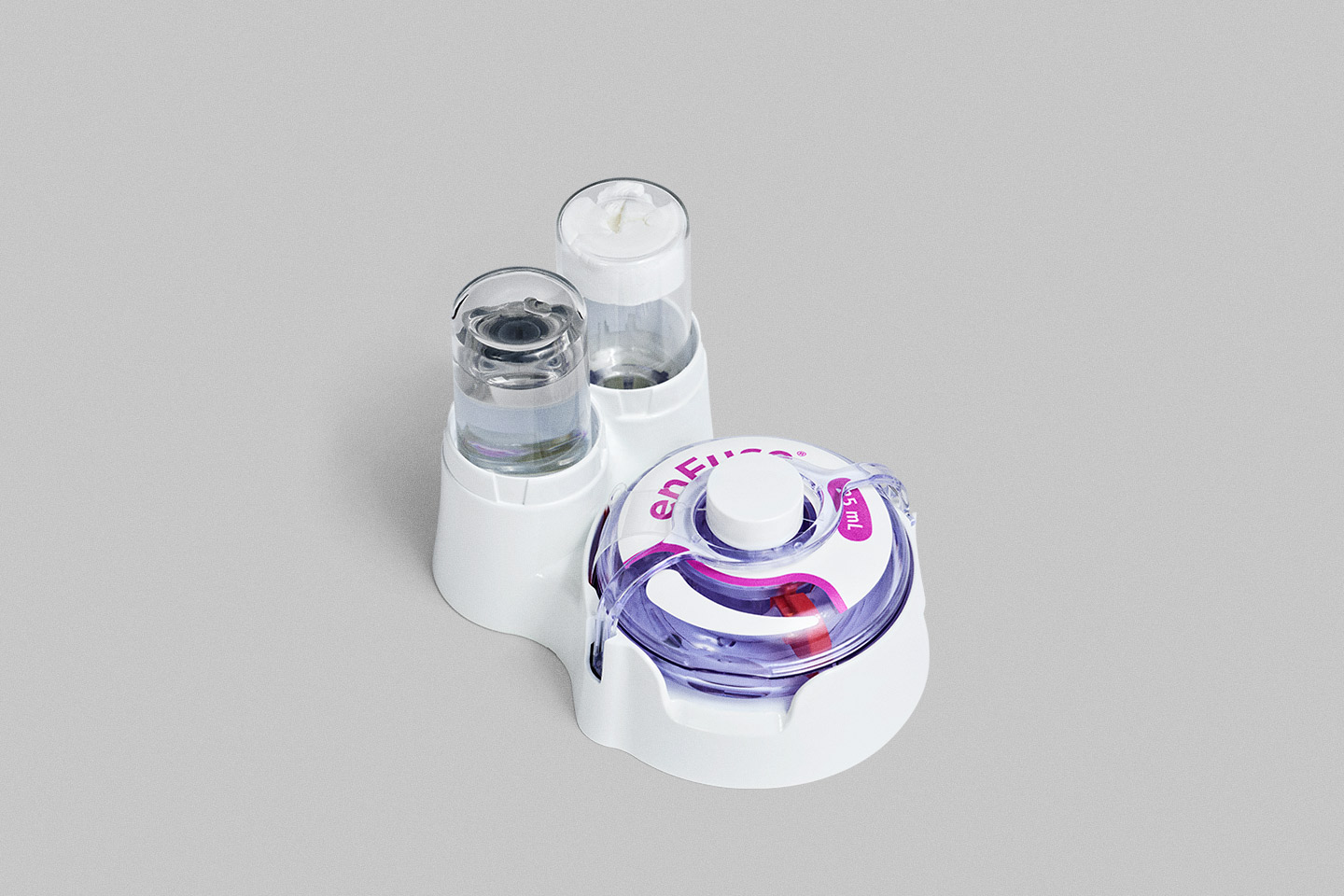
Vial Transfer System
The enFuse Vial Transfer System automates the transfer of a fixed volume of drug product from a pre-filled vial to the enFuse. Users simply insert the vial into the transfer system to load the drug product into the wearable.
Transfers a fixed volume of drug from pre-filled vials
Compatible with a range of standard vial sizes
Simple user experience to support self-administration
Commercial Simplicity
Through Automated Fluid Transfer
Original Container Closure
Both the enFuse Syringe and Vial Transfer Systems are designed to utilize original container closure, meaning that the method used to package and store drug products remains unchanged from current widely adopted vial and syringe formats.
- Eliminates the need for custom container solution
- Utilizes existing manufacturing capabilities and fill technology
- Avoids long-term stability testing associated with custom containers
- Decreases number of partners that must be managed in supply chain
- May minimize time to clinic and market2

Decrease Time to Market & Simplify The Supply Chain
Patient Experience
enFuse addresses important patient needs with easy, discreet, and comfortable drug delivery1
Hands Free
Without wiring or tubing, patients can perform light to moderate activities while medication is being administered
Hidden Needle
Featuring a small, hidden needle design, patients never see the needle before, during or after drug administration, enhancing comfort and reducing anxiety
Discreet
The small, lightweight, palm-sized wearable with no flashing lights or sounds is easily hidden under clothing during use
Comfortable
Designed to work with each patient’s own unique body composition to deliver medication through the skin as comfortably as possible
Easy to Use
The usability of the enFuse wearable and transfer systems has been proven in over 30 usability studies
Easy to Use
The usability of the enFuse wearable and transfer systems has been proven in over 30 usability studies.
Overwhelming Patient Preference for enFuse Demonstrated in Randomized Control Trial2
In a clinical trial utilizing SCIg, 100% (22/22) of surveyed patients preferred enFuse over an infusion pump primarily due to ease of use, more mobility during infusion, less setup time and less pain at the injection site.
Of all surveyed patients, 95.5% (21/22) of patients stated that they would switch to the enFuse in the future.

In Development
Enable is investing in research and development to address new patient, provider, and partner needs
Dual Vial Transfer System
The enFuse Dual Vial Transfer System will provide an automated filling system that can combine and mix drug products at the point of use.
Capable of mixing two vials of liquid drug product, or reconstituting a lyophilized powder with its appropriate diluent, the automated system is designed to avoid the complicated steps associated with traditional manual mixing processes.
Avoiding the need to measure and draw up correct volumes of diluent or drug product into a syringe, and then introduce and mix them by hand, the Dual Vial Transfer System is designed to make drug preparation safe, user friendly, and repeatable.
Potential Benefits
- Automates mixing of diluent and lyophilized drug
- Standardizes mixing process to ensure proper reconstitution
- Avoids the use of syringe and needle to manually draw drug and diluent volume
- Removes needle stick hazards and drug product exposure associated with manual mixing
- Introduces opportunities for the development of novel combination drug products
Simple Drug Preparation with Automated Mixing
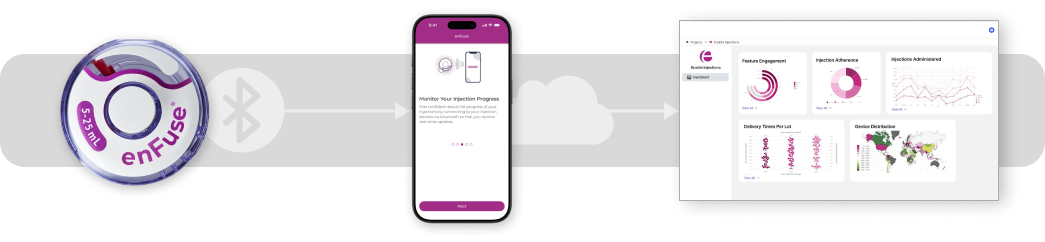
Smart enFuse
Smart enFuse
The Smart enFuse is designed to seamlessly integrate with a companion mobile application via Bluetooth to record administration event data while empowering patients with support tools such as on-boarding, real-time injection guidance, and symptom tracking. Remotely recorded adherence data and patient reported outcomes can be managed in a secure cloud and accessed via analytical dashboards in both clinical trials and real-world use.
Potential Benefits of Smart enFuse
Training Patients
Videos and interactive guidance educate the patient on their condition and therapy as well as train them on how to use the enFuse.
Gather Patient-Reported Outcomes
Electronic questionnaires help patients track key aspects of their disease treatment, providing insights for both clinical trial endpoints and post-launch disease management support
Empower Self-Administration
On-demand training and step-by-step guidance videos ensure patients understand proper enFuse use
Automate Data Collection in Trials
Real-time tracking and seamless data transfer streamline data collection, enhancing trial data quality
Drive Treatment Adherence
Recording administration date and time can identify missed or late doses, helping keep patients adherent to their treatment
Track Drug Product Use
Confirmation of the drug type and expiration ensures safe, accurate drug product usage, bolstering patient safety
Features
- Connected enFuse & Companion Mobile Application
- Automated injection event tracking
- On-demand enFuse on-boarding and guidance videos
- Injection schedule, notifications, and reminders
- Real-time injection status and progress monitoring
- Symptom and side effect journal and tracking
- Secure cloud infrastructure and data portals
Platform Customization
- Select mobile application features to align with patient needs
- Align mobile application with design branding guidelines
- Customize patient reported outcome questionnaires
- Integrate with preferred cloud infrastructure or use turnkey solution

Designed to empower patients with administration guidance and tracking tools
Manufacturing and Quality Excellence
At Enable, scalable, in-house manufacturing is a key pillar of our strategy to ensure seamless clinical and commercial supply of the products and wearable technology within our innovative enFuse drug delivery solution.
Our corporate headquarters in Evendale, Ohio currently includes GMP-compliant manufacturing space supporting manufacturing of enFuse, and Enable recently announced plans to expand our manufacturing facilitates with a new state-of-the-art 90,000 square-foot Manufacturing Center of Excellence in Springdale, Ohio.
With our new facility, Enable will be able to significantly expand and enhance production processes and ultimately reduce the time it takes to bring enFuse to patients and the medical community.
Partner With Us
enFuse was designed to overcome IV infusion shortcomings through fast, simple and convenient delivery, bringing efficiency and value to the entire healthcare ecosystem, including patients, providers, and payers, with the ability for at home self-administration.
With a design that allows compatibility with small molecule and biologic drug formulations across a wide range of viscosities and volumes (5-25mL for a single enFuse, and up to 100mL with multiple enFuse devices are worn simultaneously), we believe the applications for enFuse are limitless and offers pharmaceutical partners a unique opportunity to differentiate their drug development programs and medication offerings.
Our goal is to create long-term mutually beneficial relationships that span clinical development through commercialization. Enable continues to selectively add to its list of pharmaceutical partners, with indications and patient populations who will benefit from enFuse. If you are interested in partnering with us, please let us know by filling out our contact form.
References
1) Wasserman R, et al. IgG Exposure and Safety in Patients with PID: Randomized Crossover Study Comparing a Novel Investigational Wearable Infusor Versus the Crono Pump.” Immunotherapy. 2022 Nov;14(16):1315-1328
2) Desai et al. “Economic Benefits of an Original Container Closure During Combination Product Development: Expert-Modeled Scenarios Validated by Pharma.” Drug Delivery Summit, 28 Feb. 2024
