Large-Volume Subcutaneous Delivery
Clinical summary on the accuracy, safety, and efficacy of large-volume subcutaneous (SC) delivery
Large-Volume (LV) SC Delivery Is Safe and Effective
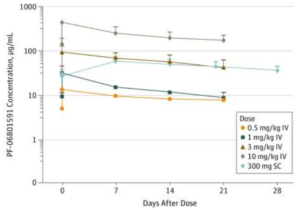
A study utilizing PF-06801591, a PD-1 inhibitor, demonstrated the following pharmacokinetic and safety data comparing IV vs SC administration (1) :
Safety: “Treatment emergent AEs across all cycles occurred in 24 (96%) patients treated with PF-06801591 IV and in 15 (100%) treated with SC administration of the drug. Grade 3 or higher AEs occurred in 11 (44%) and 6 (40%) patients treated with PF-06801591 IV and SC, respectively.”1
Efficacy: “The safety and tolerability [of IV and SC] …appear to be comparable. Exposure following SC administration was within the expected efficacious dose range.” 1
Figure 1. Concentration-Time Profiles of PF-06801591 After Intravenous or Subcutaneous Administration and PD-1 Receptor Occupancy by PF-06801591
A Permeation Enhancer Is Not Necessary for Successful LV SC Delivery
Several studies and recent approvals demonstrate that for large-volume (LV) SC delivery to be safe and effective – a chemical modifier like hyaluronidase is not necessary.
Clinical 1: “No modifications to PF-06801591 or additions such as hyaluronidase were required to enable SC administration.” (1)
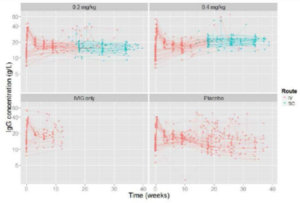
Clinical 2: No hyaluronidase (modifier) is necessary for successful SC administration of 50mL of IgG. (2)
Figure 2. Hizentra raw data plots, IgG concentration (g/L) over time (weeks) (3)
Patients Indicate Preference for Large-Volume Subcutaneous Delivery Device
The September 2022 issue of Future Medicine states: “As a wearable drug delivery system designed to automate the injection process, the Investigational Wearable Infusor* (IWI) can reduce the burden of care for patients…by helping to address some of the challenges associated with self-injection, including process complexity (IWI administration is a simpler process with fewer preparation steps and less external equipment than the Crono Pump (CP), and the IWI has a single button to operate on the infusion device), needle phobia, pain concerns, and incorrect administration, potentially optimizing disease management and outcomes.” (4)
Patient Device Preference:
“Following the final study infusion, a total of 22 of 23 patients provided responses to the device preference questionnaire, and all responders indicated that they preferred the IWI* over the CP. There were numerous reasons for preference for the IWI, the most common being that the IWI was easier to use than the CP. All but one patient indicated a preference to switch to the IWI in the future.”(4)
* Investigational Wearable Infusor (IWI) = enFuse®
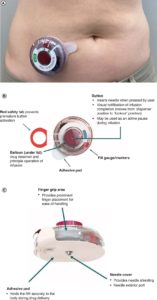
Figure 3. Overview of the Investigational Wearable Infusor (IWI) device. A: the IWI device in situ on a patient’s abdomen, B) the view from the top of the device, c) the view from the bottom of the device. (4)
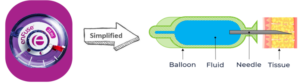
How Does enFuse® Work?
Figure 4. enFuse Large-Volume Subcutaneous Delivery Technology – Simplified
The investigational enFuse® system has not been approved for use by any regulatory agency and is currently not approved for commercial use.
Clinical Summary
- 4 Clinical Trials (3 completed, 1 ongoing) with 6 Product Configurations & 3 Pharma Partners
- >600 Devices clinically tested on >60 people worldwide
- Up to 4 devices worn simultaneously and delivered volume up to 80mL
- OUTCOMES
- Similar pharmacokinetics met in all studies with no safety issues (5)
- The rate per infusion of most TEAEs, including injection site reactions, was numerically lower when SCIg was given via IWI (enFuse®) rather than an infusion pump
- 100% of surveyed patients prefer IWI (enFuse) over other Large-Volume Subcutaneous Pump.
To learn more, watch a 1-minute video on enFuse, or contact us at EnableInjections.com/contact
Sources:
- Johnson, M., et al. “Assessment of SC vs IV Administration of Anti-PD-1 Antibody PF-06801591 in Patients with Advanced Solid Tumors, a Ph 1 Dose Escalation Trial.” JAMA Oncology, 30MAY2019. (link)
- Hizentra Label CSL Behring. FDA.gov/media/78466/download. (link)
- Hizentra Variation Assessment Report, EMEA – ema.Europa.eu (link).
- Wasserman R, et al. “Systemic IgG exposure and safety in patients with primary immunodeficiency: a randomized crossover study comparing a novel investigational wearable infusor versus the Crono pump”. Immunology. 21 SEP 2022, Future Medicine. https://www.futuremedicine.com/doi/10.2217/imt-2022-0097
- Data on file, Enable Injections, Inc.
