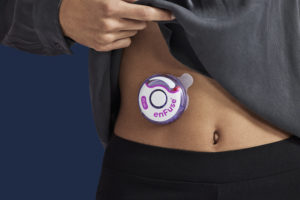
New Look, Same Mission – enFuse®
Enable Injections is excited to introduce the new look for the enFuse® lid. The new enFuse lid design has an improved user interface with a streamlined dosing window and an enlarged area for branded product information.

The enFuse® Advantage – Flexibility
Enable Injections’ enFuse® accommodates delivery of drug and biologic products in large volumes ranging from 5 – 25 mL. Once approved, enFuse will allow EnywhereCareTM – enabling patients and healthcare providers to choose the delivery setting, ranging from in-clinic, to remote, to at-home self-administration.
The Impact of Innovation and Subcutaneous Delivery
For many patient populations, long-term treatment is imperative. For these patients, innovation has the potential to make a great impact.
One lead study investigator discussed Phase 1b results of clinical findings at the June 2022 annual meeting of the American Society of Clinical Oncology (ASCO 22)1, saying, “There is a great need among these difficult-to-treat patients for safe and effective treatment options that can delay the progression of the disease while preserving their quality of life.”
In the interview, the lead study investigator acknowledged the uptake of changing treatment methods could be a challenge to switch to subcutaneous (SC) administration while the current standard is IV therapy. However, “once adopted, this method of administration could significantly minimize the amount of time both patients and their physicians spend over the course of treatment,” the lead study investigator told The Pharma Letter. The investigator shared that the product “represents a novel potential paradigm for RRMM patients.”
Asked about SC administration, the lead study investigator said: “subcutaneous administration, and particularly using an OBDS, would allow patients to experience the benefits of treatment with [the drug] with greater convenience and less time spent receiving therapy.”
To learn more about enFuse flexibility and partnering with Enable Injections, contact us.
Resources
1. Simon Wentworth. ‘ASCO 22 interview: Sanofi innovates in multiple myeloma’, The Pharma Letter. 06 July 2022. https://www.thepharmaletter.com/article/asco-22-interview-sanofi-innovates-in-multiple-myeloma
