Advantages of Subcutaneous Drug Delivery
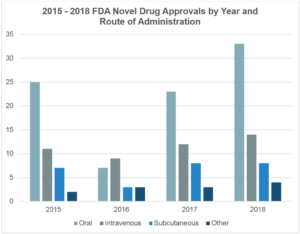
Innovation in medicine has led to the development of novel small molecule and biologic formulations that require parenteral administration via intravenous (IV), subcutaneous (SC), intramuscular (IM), or other administration routes via injection. Among these, biologics subcutaneous delivery has gained prominence due to its patient-friendly approach. Concurrently, innovative drug delivery systems (e.g., infusion pumps, autoinjectors, wearable infusors, etc.) have also been developed to facilitate patient access to parenteral therapies. Accordingly, the number of novel small molecule and biologic drug products approved for parenteral administration have risen in recent years (Figure 1), including drug products for subcutaneous administration. There were 95 biologic therapies for subcutaneous administration approved by the FDA in 2017 alone and an approximate 240 biologics for subcutaneous administration are in development or in process for approval by the FDA1.
It’s important to note that SC medication administration, subcutaneous injection drugs, SC injection, and SQ injection all refer to the practice of administering medication into the subcutaneous tissue.
The Adaptation of Subcutaneous Therapeutics Administration and Technology
IV administration requires access to a medical facility with trained medical professionals, which is prohibitive to many patients due to the distance or travel to medical facilities. IV administration typically requires a trained medical professional to set-up an IV infusion set and determine appropriate dosing/infusion rate and time. In addition to the risk of infection at the injection site, IV administration is also associated with the risk of systemic infection, potential exposure to IV particulate matter, and exposure to hospital-acquired infections.
For IV administration, additional time is required before, during, and after the actual infusion for the preparation, infusion duration, and observation post-infusion. Because of the extended time, supplies, facility, trained staff, and equipment required for IV drug administration, IV therapeutics generally have an increased cost versus other methods of administration2.
Subcutaneous Administration
SC administration has traditionally been limited by the volume which can be delivered (<1 to 2 mL), injection site degradation of the therapeutic (absorption), and dose range. But with recent advances in formulation and delivery technologies, SC administration is becoming more adept at administering a wide variety of therapeutics.
Subcutaneous administration allows therapeutics to be self-administered by patients or healthcare providers using a variety of different drug delivery systems, including advanced subcutaneous drug delivery devices. Because SC administration facilitates patient self-administration in home or outpatient clinical environments, SC administration provides a reduction in medical facility fixed costs. In a 2015 meta-analysis evaluating socio-economic impact of SC versus IV administration of trastuzumab for HER2 positive metastatic breast cancer, it was estimated that the use of SC administration could contribute to a cost saving between 758 and 2576 euro per annual course, which is an estimated savings of 1.4 to 1.6 million euros per year in Belgium3. SC administration was also reported to a 3-time reduction in the total preparation and administration compared to IV administration3. When considering subcutaneous vs intramuscular injection routes, subcutaneous injections often result in less discomfort and are easier to administer, enhancing patient compliance.
SC administration provides flexibility in the anatomical infusion site, and options include the stomach, thighs, and backs of the arms. Subcutaneous infusion systems can be designed with smaller needle sizes, which may decrease pain during infusion. While the risk of infusion site infection still exists with SC administration, infusion site infections are generally limited to cellulitis and very rarely progress to systemic infections. Further, administration at home reduces the risk of exposure to hospital-acquired infections.
In a 2015 meta-analysis 4, a literature search was conducted to identify clinical studies published from 1980 to February 2015 that included comparison of IV, IM, or SC administration in order to determine the advantages and disadvantages of each administration route and investigate patient preference. In studies comparing SC to IV administration, patients reported a higher preference for subcutaneous administration (88.9%) versus IV (9.6%). An international, randomized, two-cohort study (PrefHer) reported similar results, in which 92% of patients stated they preferred SC administration of trastuzumab versus 8% for IV administration.3
In addition, a systematic review of randomized, controlled trials and/or crossover studies investigating patient preference reported that patients preferred SC versus IV administration in four of 6 trials5. In addition, a non-interventional time and motion study was conducted in 2016 in eight countries to determine time savings with rituximab injection via SC versus IV infusion. The study determined that patient SC self-administration at home decreased treatment time 6.
Enable Injections’ enFuse® on-body infusor is limited by Federal (or United States) law for investigational use only and is designed for subcutaneous administration of large-volume parenteral drug products by patients or their caregivers.
To learn more about the enFuse drug delivery device, subscribe to our monthly newsletter with the latest in enFuse News. For pharma partners with inquiries into pairing a therapeutic with the enFuse for development, contact us.
Resources:
- Roots Analysis Private Limited. “Subcutaneous Biologics, Technologies and Drug Delivery Systems (2nd Edition), 2018 – 2030.” 2018.
- Schmier, Jordana, et al. “Costs of Providing Infusion Therapy for Rheumatoid Arthritis in a Hospital-based Infusion Center Setting.” Clinical Therapeutics, Volume 39, Number 8, 2017, https://www.clinicaltherapeutics.com/article/S0149-2918(17)30731-2/pdf. Accessed 20 NOV 2018.
- Papadmitriou, K, et al. “The Socio-economical Impact of Intravenous (IV) Versus Subcutaneous (SC) Administration of Trastuzumab: Future Prospectives,” Facts Views Vis Obgyn, 2015; 7(3): 176-180, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4788333/. Accessed 22 JAN 2019.
- Jin, Jing-fen, et al. “The Optimal Choice of Medication Administration Route regarding Intravenous, Intramuscular, and Subcutaneous Injection.” Patient Prefer Adherence, 02 JUL 2015, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4494621/. Accessed 22 JAN 2019.
- Patient, 12 JUL 2014, https://www.ncbi.nlm.nih.gov/pubmed/25015302. Accessed 20 NOV 2018.
- De Cock, Erwin, et al. “Time Savings with Rituximab Subcutaneous Injection versus Rituximab Intravenous Infusion: A Time and Motion Study in Eight Countries” PLOS ONE, 30 JUN 2016, https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0157957. Accessed 21 JAN 2019.
Author:
Jennifer King, Marketing Manager, Enable Injections
