Sustainability Improvements in Drug Delivery with Allison Mack
The carbon footprint of an IV infusion may be more than you think. Hear more from Allison Mack, Medical Education Lead at Enable Injections, about the waste comparison of a standard IV and the enFuse systems in our latest #EnableWearableDelivery executive video series!
Transcript below:
Hi, my name is Allison Mack and I’m the nurse practitioner medical education lead here at enable injections today. I’m going to be talking to you about sustainability and IV waste comparison between IV infusions and our device.
So when people associate an IV infusion or IV equipment a lot of times they think well, I just need a basic bag of meds and a needle. Well, in fact, that’s not the case. You do need a lot more supplies when it comes to inserting an IV.
Here, we have the poll the bag the tubing and extension tubing and all of supplies here listed on or shown on the table. So you have your basic tape your syringe to flush lines. You have to have a barrier here to protect patient or the table that you’re going to be setting yourself up on you have your alcohol wipe shoot bandage and your tag Durham. This is all single-use equipment and it is set up for just one successful IV insertion.
We all know that IV’s never go in the first time easily. There are often multiple attempts and when that happens you actually need more equipment that’s listed here. You’re going to need a new needle you’re going to need more than one alcohol wipe and you’re definitely going to need more bandages.
Keep in mind as well with this equipment laid on the table. Every one of these items is single use so they come individually wrapped. So these are the bags here just to highlight some of the material that each and every single one of these come in they never come in as a kit.
So, what we have here too is a 3D model of some of the waste that’s laying on the table here tied to a bottle here. So, it just goes to show that putting an IV is just simply one needle in one bag. There’s a lot of other things that are involved going into an IV insertion and in infusion.
On the other side. We have our device the end views device. We have two devices here the gas file and the syringe instead of just one IV insertion kit. We actually have two devices here and you can clearly see we still have less equipment less waste than you do for a basic IV. We just have the devices the bases each every time you apply one of the devices you need a single-use alcohol wipe, you need your vial of medication and a syringe depending on the device that you use and each one comes in their carton. that houses the device this is resource none of this may or may not be used depending on where the device is actually placed if it’s placed in the home a lot of times the user the patient themselves may choose not to use gloves if it’s placed in a facility then they standard standardization is typically with gloves. So bottom line is IVs, although they may seem simple and produce less waste that’s not the case at all.
The enFuse® itself is clearly showing less wastage, and hopefully it’s a carbon footprint reduction.
The investigational Infuse system has not been approved for use by any regulatory agency and is currently not approved for commercial use to learn more about the Infuse. Please visit enable injections.com.
Reduce Pharma Development Time: Large-Volume Subcutaneous Delivery with the enFuse
Subcutaneous (SC) drug development presents several challenges, and these challenges are amplified when trying to achieve a low-volume formulation. Intravenous (IV) administration has a bioavailability of 100%, but the bioavailability for SC monoclonal antibody drugs is 60%–80%. This means the dose is typically higher for SC delivery than for IV delivery to achieve therapeutic efficacy.
Unfortunately, there is a common and inaccurate belief that SC drug delivery should not exceed a volume of 3 mL per dose. Several well-known drugs are available subcutaneously at volumes >3 mL, including, but not limited to, trastuzumab, rituximab, daratumumab and pertuzumab/trastuzumab. Despite these therapies being co-formulated with a permeation enhancer (hyaluronidase), they are still administered at quite large volumes, ranging from 5–15 mL and delivered over several minutes. With current innovations, it is unnecessary for manufacturers to continue attempting to overcome physiochemical challenges to achieve a small volume (<3 mL) for SC delivery. Formulation challenges can be expensive and time consuming, delaying convenient life-saving treatments for patients.
The process for storing a drug for SC delivery is multifaceted and involves several steps. The container that the drug is stored in can vary and requires substantial testing, but most often, biologic drugs are manufactured and stored in glass vials. From beginning to end, this testing is expensive and can take several months; therefore, the chosen SC delivery method can impact time to market, development costs and even commercial uptake. The value of reaching the market faster with a flexible drug delivery format is an obvious benefit and even more pronounced when considering the evolving competitive landscape and limited patent life of a molecule.
While the drug is the foundation of treatment, the delivery method of the drug is critical for patient preference, as some methods of SC delivery allow for self-administration at home. Various methods exist for delivering drugs and they commonly include prefilled syringes, autoinjectors, infusion pumps and, more recently, on-body delivery systems (OBDS). However, larger volumes are administered by infusion pumps or OBDS, like the enFuse, and allow flexibility for administration at home or in the clinic.
These methods vary in complexity of development due to certain requirements with container and formulation stability. This article focuses on the impact of formulation changes and container closure systems on development time, as well as touching on patient preference.
Dave Kroekel Promoted to Senior Vice President, Product Development & Operations
We are happy to announce Dave Kroekel’s promotion to Senior Vice President, Product Development & Operations, and newest member of the Executive Team. In Dave’s new role, he will continue to be responsible for Product Development and will now also have responsibility for Manufacturing, Supply Chain, and all Operations.

In June 2020, Dave joined Enable as VP, Product Development, and under his leadership we’ve successfully completed design verification and validation, as well as manufacturing readiness activities.
David Kroekel has over 35 years of experience in medical device and combination product operations and product development. He joined Enable from DSM Biomedical, where he was most recently the Biomedical Global Head of Operations, responsible for global leadership of biologics and medical materials manufacturing. Dave previously served as the Innovation Director, where he led new product development and innovation.
Prior to DSM, Dave held various senior positions in the medical device industry as Vice President of R&D for Flowonix Medical and Teleflex Medical, as well as the Sr. Director of R&D for Arrow International’s EMEA business in Belgium and the Czech Republic. Dave earned a Bachelor of Science in Mechanical Engineering from the Pennsylvania State University and a Master of Science in Manufacturing Systems Engineering from Lehigh University.
Congratulations, Dave!
Sustainability Improvements in Drug Delivery: A Waste Comparison of IV vs. enFuse Large-Volume On-Body Delivery System
Climate change has been a concern on a global stage for years now and has been called “the biggest global health threat of the 21st century” by the British medical publication The Lancet.1 Despite health care’s mission to protect and heal, the industry as a whole is contributing to the excess of emissions and increase in global waste. However, implementing small changes has the potential to have a lasting impact around the world.
Health Care’s Role in Sustainability
Our environment plays a huge factor in public health, and yet, the health sector is contributing significantly to climate change. Through building construction and operation, energy and resource consumption, and waste generation, health care operations are a significant source of carbon emissions around the world. Health care’s climate footprint is equivalent to 4-5% of global net emissions, which would be the fifth-largest emitter on the planet if it were its own country.2,3 As a result of the COVID-19 pandemic, the levels of medical waste has increased up to 10 times in healthcare facilities according to a WHO spokesperson.4
Health care systems and the medical community must balance ensuring safety for each patient while also considering sustainability for the world. Certain things cannot be reused or recycled due to bio and hazardous waste, however, the medical community can work to reduce overall medical waste where possible.
More Supplies, More Waste
The general public may not realize the amount of supplies needed to care for even one patient, as hospitals generate over 29 pounds of waste per bed per day.5 For example, many people think of IV treatment as an IV bag, tubing, and a needle. In reality, a lot of supplies go into the entire process to ensure safety for the patient, including gloves, alcohol wipes, gauze, towel, bandage, syringe, medication bag, IV bag hanger, and an IV administration set with bag spike and connector, which are all single-use and non-recyclable. Not only does more supplies mean more waste, but it can also be cumbersome for health care workers to carry and organize. Plus, it can be overwhelming for patients to see the amount of supplies necessary for one treatment.
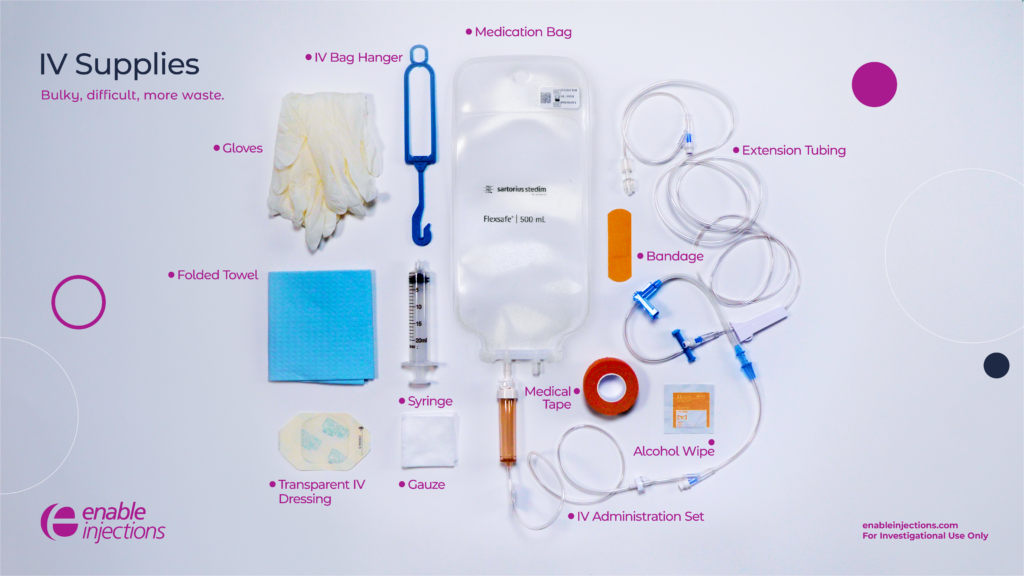
All these supplies are needed for a single IV administration and must be repeated for each patient and each administration. Additional supplies may also be needed if the initial IV insertion fails, causing the health care provider to repeat steps and supplies.
IV Waste vs the enFuse
The novel enFuse delivery system is a compact solution for delivering drugs potentially more efficiently and with reduced waste compared to traditional IV administration. The enFuse system can deliver high volumes of therapeutics, from 5-25 mL with a single device, and could potentially replace IV treatment for many medications, especially treatments that are needed for chronic conditions.
Rather than the large medication bag and the extra materials like bandages and gauze, the enFuse system utilizes an injector device, a transfer base (either syringe transfer or vial transfer), a small plastic tab, and an original medication vial. Gloves are used as a precaution only by health care professionals.

While the enFuse is a single-use device and must be disposed of properly after use, the overall amount of waste, when calculated by weight in grams, is reduced by using either the enFuse syringe transfer system or the enFuse vial transfer system compared to traditional IV administration.
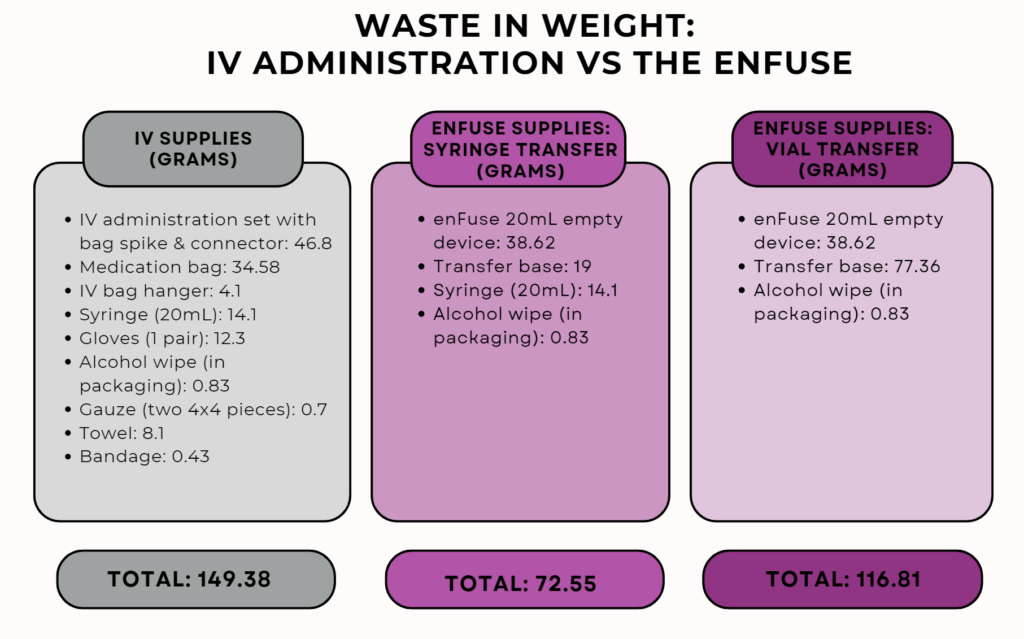
Reducing Medical Waste with the enFuse
By replacing standard IV administration with the enFuse system, health care could reduce certain medical administration waste by 51% with the enFuse syringe transfer system and 22% for the vial transfer system according to the weight measured for each administration method.
The enFuse is designed to allow for self-administration at home by the patient, which could cut down on transportation needs for patients to and from a health care facility, as well as healthcare professionals’ time and capacity.
While infusions are one of many contributors to health care’s climate footprint, a small improvement can compound into a significant lasting change in the future.
References:
- The Lancet. (2009). A commission on climate change. The Lancet, 373(9676), 1659. https://doi.org/10.1016/s0140-6736(09)60922-3
- Watts N, Amann M, Arnell N. The 2019 report of The Lancet Countdown on health and climate change: ensuring that the health of a child born today is not defined by a changing climate. Lancet. 2019;394:1836–1878.
- Karliner J, Slotterback S, Boyd R, Ashby B, Steele K. Health Care Without Harm; Reston, VA: 2019. Health care’s climate footprint. How the health sector contributes to the global climate crisis and opportunities for action.
- Bateman, K. (2022, February 17). Covid-19 has caused a surge in plastic medical waste. Here’s what needs to be done about it. World Economic Forum. Retrieved December 6, 2022, from https://www.weforum.org/agenda/2022/02/medical-waste-plastic-environment-covid/
- Waste: Understand hospital waste streams, how to measure them, and how to reduce waste at your facility. . Practice Greenhealth. (n.d.). Retrieved December 6, 2022, from https://practicegreenhealth.org/topics/waste/waste-0
The Real Role of Regulatory Affairs
Our next video in our Enable Wearable Delivery executive video series features Russ Pagano, Vice President of Regulatory Affairs & Quality. Tune in to learn more about how regulatory departments act as salespeople for a company!
Transcript below:
Hi, I’m Russ Pagano. I’m the VP of Regulatory and Quality here at Enable Injections, and I’m here today to talk a little bit about regulatory and the regulatory pathway for our products.
When people think of regulatory, typically they think of something restrictive, something binding where we say no a lot. We tell people you can’t do this, you can’t do that. However, I look at us as being salesmen. We’re really the first line of salespeople for the company. And why do I say that? Well, because when you think about it, Enable’s goal is to really treat and help patients as much as we possibly can. But in order to get a product to patients, we have to go through a number of customers. A number of customers have to buy off on our technology. They have to agree that it’s safe and effective.
So, who’s our first customer? Our first customer is obviously ourselves. We need to make sure that we are perfectly comfortable with the product, that the product is going to be safe, effective, it’s going to work, it’s going to do what it says it does, and it’s going to really help people. Otherwise, what’s the point of doing what we do?
The next couple of customers are slightly different than you would think. They’re not really hospitals, it’s not doctors. Those are the end customers, if you will, who then treat their patients and work with their patients, with the product. Your initial customers are really the big drug companies and the regulatory authorities.
So the big drug companies, we have to convince them that we’re safe and effective, otherwise they’re not going to partner with us. They’re not going to want to use our product with their drug. Obviously these drugs are worth billions of dollars, some of them, and they want to make sure that they’re being able to apply that and send that to customers in a manner that is going to be an improvement over what they’ve currently done. So, we need to sell that. What’s the first question that big drug companies to ask us: Are you approved? Can you sell your product in the market? And right now, our answer is no, but we are rapidly on our way heading towards approval.
And how does that work? Well, that works through the company, with regulatory taking some of the lead as selling to the FDA, the Food & Drug Administration, to the European market, the European Medicines Agency, we need to sell our product and convince them that we’re safe and effective. All the work we do behind the scenes, all the testing, all the design development, all of that comes out and has to be presented in a manner such that an intelligent, scientific person who knows nothing about the product can now take it, see it, and say yes, this is something that the American people or the European folks, or the rest of the world, should have. Through that, once they are okay and once they approve us, then we turn it over to the sales and marketing force and others who then go out and sell to hospitals, etc. and convince the hospitals that this is a good thing for your patients. And hopefully at the end of the day, we have a bunch of happy patients. Thank you.
The investigational enFuse system has not been approved for use by any Regulatory agency and is currently not approved for commercial use.
Enable Injections Enhances Board with Addition of Nigel Sheail
New director, Nigel Sheail, adds global biopharma experience to Enable Injections, strengthening its mission to lead drug delivery innovation worldwide.
CINCINNATI, OH—November 10, 2022 /PRNewswire/ – Enable Injections, Inc. (“Enable”), a company developing and manufacturing the enFuse® platform of investigational wearable drug delivery systems, is pleased to announce the appointment of Nigel Sheail to the company’s Board of Directors, effective October 27, 2022.

Nigel brings over two decades of expertise as a global biopharma executive, most recently at Novartis as the Global Head of Mergers & Acquisitions and Business Development & Licensing. Prior to Novartis, Nigel worked at Bayer Healthcare AG, F Hoffmann-La Roche Ltd., and GSK.
“Enable Injections’ innovative delivery technology, enFuse, has the potential to positively impact the patient experience and support healthcare providers to optimize their practice,” said Nigel Sheail. “I look forward to working with the Enable Injections management team and Board at this exciting time in the company’s development.”
“We are delighted to welcome Nigel as a new director to Enable’s Board,” said Mike Hooven, Chairman and CEO of Enable Injections. “His business experience with global pharmaceutical companies brings new perspectives and skills which will be critical as we grow our business into a major player in the healthcare and drug delivery industry.”
The appointment of Nigel increases the size of Enable’s Board to nine members following other recent appointments this summer in June and August, adding meaningful operating, investing, pharmaceutical, and delivery systems experience to Enable Injections.
Enable Injections’ enFuse® is a hands-free, hidden needle, large-volume drug delivery technology designed to subcutaneously (SC) deliver therapeutics via constant pressure.
View the original release on PR Newswire.
Enable Injections Commits to Cincinnati Growth with Increased Jobs
Enable Injections reinforces commitment to Cincinnati region with job and payroll expansion through state tax credit program and support from state organizations.
CINCINNATI, October 31, 2022 /PRNewswire/ — Enable Injections, Inc. (“Enable”), a company developing and manufacturing the enFuse® platform of investigational wearable drug delivery systems, is pleased to announce the commitment to increase jobs and payroll in the Cincinnati region with an additional 257 jobs and $19,865,376 in payroll by 2027.
Approved today by the State of Ohio’s Tax Credit Authority Board, Enable will benefit from Ohio’s Job Creation Tax Credit (JCTC) program because of its commitment to the Cincinnati region and State of Ohio.
“Enable Injections has experienced strong growth in the Cincinnati area since 2010, developing the enFuse, an innovative drug delivery technology which has the potential to positively impact patient experience worldwide. Enable Injections is proud to be established in Cincinnati and is on a clear path to create more high-tech, high-value jobs and opportunities for our city and State of Ohio,” said Mike Hooven, President and CEO of Enable Injections. “We are relentless in our pursuit of transformative and impactful drug delivery technology, and in our commitment to expand our presence in the region.”
“Cincinnati is a great place to do business,” said Tim Flaherty, Chief Financial Officer at Enable Injections. “The cost of living and doing business in Ohio are advantageous, especially compared with other parts of the country. The support businesses receive from the Ohio economic development organizations and their incentive programs continue to help Enable Injections develop and thrive with talented staff.”
Not only will Enable Injections add jobs to the city, but also retain 193 jobs and $20,389,023 in payroll. The JCTC and pending additional support from JobsOhio could potentially total more than five million ($5MM) in funding, allowing Enable Injections to invest in further development in the Cincinnati area. The State of Ohio, JobsOhio, and REDI Cincinnati all provided support for the project.
“Enable Injections is an innovative leader in healthcare technology that has experienced tremendous growth in Ohio,” said J.P. Nauseef, JobsOhio president and CEO. “Its expansion and creation of hundreds of new jobs in the Cincinnati market over the last 11 years reaffirms the strong partnership that Ohio and Enable Injections have built due in part to the competitive advantages businesses find here, including a highly-skilled workforce and affordable cost of doing business.”
“Enable, which has roots within our partner CincyTech’s portfolio, has been on a rapid expansion since 2015 and we are proud to support its growth in the Cincinnati region,” said Kevin Donnelly, REDI Cincinnati’s vice president for project management. “This success can be attributed to several factors, exceptional products and strong company leadership from Mike and Tim, a region that fosters growth due to access to STEM and manufacturing talent afforded through our robust educational environment and support from a business community that was recently ranked a top-25 national tech hub by BestColleges.”
Since 2010, Enable Injections has continued to grow in the Cincinnati region, with locations in Evendale, West Chester and Franklin. By offering a unique solution through patented elastomeric technology, Enable Injections is positioned to offer patients the flexibility of in-clinic or at-home self-administration so they can focus on what matters most to them.
View original release on PR Newswire.
Thinking Clinically: Potential Benefits with the enFuse
Our next video in our Enable Wearable Delivery executive video series features Mehul Desai, Vice President of Medical Affairs. Tune in to learn more about potential clinical benefits for the enFuse!
Transcript below:
I’m Mehul Desai and I’m the Vice President of Medical Affairs at Enable Injections. I’m a pharmacist by training with a background working on treatments for rare diseases all of which were intravenous or subcutaneous therapies.
After seeing and experiencing the limitations of current drug delivery routes throughout my career, the enFuse technology resonated with me.
Current drug delivery routes such as intravenous, syringe, syringe pumps and autoinjectors have limitations including a visible needle, volume restrictions and hands-on administration among others.
One of the most exciting potential benefits around the enFuse technology is the potential to demonstrate clinical benefit. A unique aspect of the technology is the use of a constant-pressure design utilizing elastomeric technology rather than a constant-flow design using an electromechanical pump. This design allows the enFuse to automatically adapt to the injection site back pressure, and it is hypothesized to potentially alleviate infusion site leakage and pain.
Among other benefits, the enFuse has a hidden needle and provides flexibility. About a quarter of the adult population demonstrates a needle phobia; fortunately, the needle is hidden the entire time the patient handles the enFuse so this design has the potential to positively impact adherence. As for flexibility, the enFuse is a hands free administration so this could increase flexibility by allowing treatment in a clinic or at home.
With these differentiating factors, we believe that the enFuse technology has the potential to revolutionize drug delivery and enable wearable delivery for patients.
The investigational enFuse® system has not been approved for use by any regulatory agency and is currently not approved for commercial use.
Flexible Care – Enable Wearable Delivery
The following excerpt is originally published at ONdrugDelivery September 2022 issue, linked here.
FLEXIBLE CARE – ENABLE WEARABLE DELIVERY
Anne heard the news from her physician at a young age – she had a chronic disease, one for which there is no cure. She had few options – she could either ignore her diagnosis and live confined to the full progression of her disease or she could aim for remission and learn to live with needles, nurses, an infusion chair and a rigid infusion schedule. Anne did not feel as if she had a choice.
After a few years of intravenous (IV) therapy, Anne found out she was in remission, and was elated. To stay in remission, she continued her therapy, but a change in policy by her insurance company meant she could no longer receive her IV infusions in the clinic. She had to move to home health for her IV infusions. Even though getting IV therapy at home is good news for many patients, as they no longer have to commute, Anne felt as though it was one more thing that she could not control.
The home infusions did not go as planned. The first scheduled home infusion day came, but the medicine and supplies didn’t arrive until the next day. The rescheduled first home infusion day also did not work out, as a different home health nurse got lost and never arrived at her home. The third try was a success, but Anne had new concerns. What happens when the nurse is different every time? And what about the risk of covid-19 exposure by allowing a nurse who had visited other patients that same day and week? Was she comfortable having a stranger in her home every time she needed her infusion?
She realised that, as an immune-compromised patient, she did not want to be exposed to health risks like covid-19 in her home or share her couch and TV shows with a stranger while she received her IV treatment. It was such a concern that Anne informed the insurance company she wanted to go back to the IV clinic because she had more control. The insurance company refused the request, and Anne compromised by agreeing to receive her infusions in the home health company office building. The room where she received her IV therapy was a remote, unfriendly cinder block room. It was not what she wanted, but she felt she did not have a choice. Was there an alternative?
GAP IN CURRENT CARE
For patients and healthcare providers (HCPs), the subcutaneous (SC) option is a strong one. However, many drugs are not available for SC delivery, for a variety of reasons.
Parenteral Administration
Many drugs must be administered parenterally, specifically biologics, and a common parenteral route is via IV administration. IV administration requires an HCP to initiate and monitor the infusion, usually takes several hours and involves an exposed needle.
SC administration, another parenteral route of administration, requires a fraction of the time of IV administration. However, SC administration has traditionally faced limitations, such as drug volume limitations, safety concerns due to exposed needles, hands-on administration and lack of mobility during administration.
Drug Volume Limits
SC administration has historically only involved small volumes (<5 mL) of drug delivered by a device through a needle – such as prefilled syringes and autoinjectors. To reach larger delivery volumes (>5 mL), dosing regimens would have to include multiple injections by a syringe or autoinjector per dosing time.
For pharmaceutical formulation teams, volume restrictions are an impediment. By allowing formulations to be delivered in larger volumes by using modern large-volume SC delivery technology, the development time may be reduced (Figure 1).
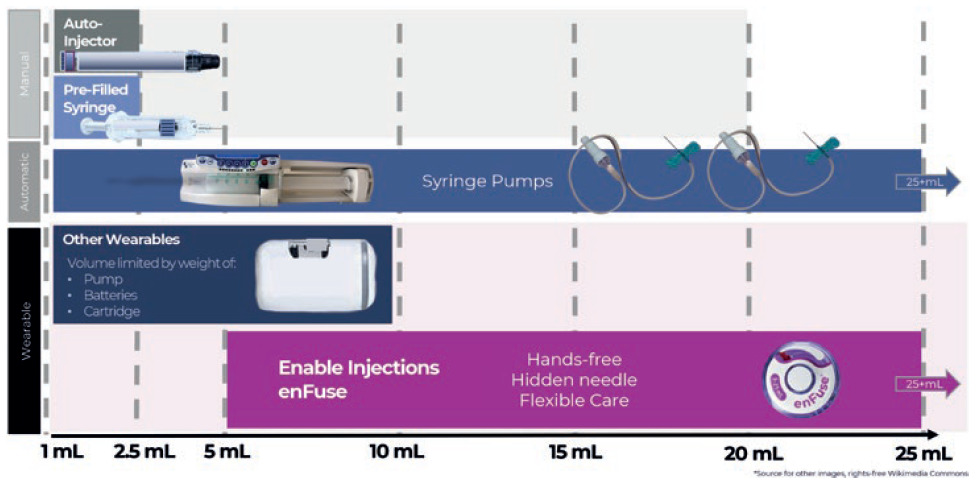
Figure 1: Overview of subcutaneous drug delivery system technology by delivery volume capacity, by type of administration for manual systems and wearable systems.
Exposed Needle Safety Concerns
Dislike of exposed needles is an issue that affects much of the population. In research conducted in a validation study with kidney failure patients receiving treatment, 26% reported feeling afraid, 41% reported feeling nervous and 33% reported feeling worried the “moment the nurse comes to insert the needle”.2
Studies report that approximately 25% of the adult population has a fear of needles, and this percentage is higher among children and adolescents.3 A 2021 review of management of needle fear in adults with chronic disease discusses a study in which 35% of patients reported delaying treatment because of needle phobia due to fear of pain.2
Hands-Free Needs
For small- and large-volume delivery via syringe, the medicine must be delivered to the patient using a hands-on approach. For current large-volume SC delivery via syringe, an HCP sits inches from a patient’s body, actively pushing the barrel of a large-volume syringe. This syringe delivers therapeutics through an exposed needle stuck into the subcutaneous tissue of the patient’s stomach, and typically requires 5-10 minutes of pushing. The focused effort required for this type of injection limits the HCP’s time, and the physical rigour required to administer this type of large-volume injection limits the number of times an HCP can repeat the process in a single day.4,5,6
Large-Volume Delivery Alternatives
One alternative to the large-volume delivery with a syringe is a syringe pump. A large-volume syringe pump removes the need for an HCP to manually inject the medication into the SC tissue. However, the pump does not eliminate exposed needles, tubing sets or the need for the patient to sit immobile throughout the infusion.7
Improvements in Large-Volume Delivery
On-body delivery device volumes are usually limited by device weight, size and container. Current on-body delivery devices are limited to volumes of 10 mL and below. These devices deliver volumes that may also be administered by several self-administered prefilled syringes, autoinjectors or large-volume syringes administered by an HCP.
IMPROVEMENTS THROUGH INNOVATION
Innovations in healthcare are imperative for improving access to medicines and the delivery of therapeutics for patients, caregivers and providers. For patients seeking flexible, convenient care that saves time and fits into their schedules, SC delivery has that potential with large volume, on-body delivery technology that is truly innovative in size, shape and wearability. As one key opinion leader said at the American Society of Clinical Oncology (ASCO) congress in Chicago, IL, US, June 2022, “SC administration by on-body delivery systems is well tolerated, requires short duration of injection and provides a convenient hands-free option.”8
Innovation benefiting patients and providers has the potential to increase flexibility and:
- Reduce needle exposure
- Allow ease of treatment and hands-free administration
- Allow potential flexibility in the location of the administration – in the clinic or at home, based on the patient and provider needs and preferences.
enFuse Enables Flexibility for Pharma
Join our mission to redefine drug delivery with our new executive video series, Enable Wearable Delivery. The first video, “enFuse Enables Flexibility for Pharma” features Enable Injections’ CEO Mike Hooven discussing the flexibility of the enFuse system.
Transcript below:
The enFuse delivery system is capable of delivering large volumes of subcutaneous therapeutics. enFuse is unique in the drug delivery space because it is fully mechanical and is designed to be simple, lightweight, and offer flexibility. This flexibility the enFuse offers is one of the greatest advantages.
The enFuse is capable of delivering any volume from 5 to 25 mL.
The enFuse also offers flexibility for the place of delivery, whether in a hospital or clinic, in CVS or Walgreens or at home by a healthcare provider or self-administered by the user.
Enable Injections provides our Pharma partners the ultimate flexibility in the manufacture and distribution of their drug because enFuse utilizes the original container closure. The container, the manufacturing process, storage or the distribution of the drug does not need to be modified in order to utilize the enFuse.
enFuse can be paired with either a vial transfer system or a syringe transfer system for transfer of drug from the original container into the enFuse at the time of use.
The vial transfer system transfers the entire contents of the vial automatically into the injector. The syringe transfer system is ideal for weight based dosing or variable dosing and is ideal for clinical studies.
To learn more about the enFuse wearable drug delivery solution, contact us.
The investigational enFuse® system has not been approved for use by any regulatory agency and is currently not approved for commercial use.