enFuse Enables Flexibility for Pharma
Join our mission to redefine drug delivery with our new executive video series, Enable Wearable Delivery. The first video, “enFuse Enables Flexibility for Pharma” features Enable Injections’ CEO Mike Hooven discussing the flexibility of the enFuse system.
Transcript below:
The enFuse delivery system is capable of delivering large volumes of subcutaneous therapeutics. enFuse is unique in the drug delivery space because it is fully mechanical and is designed to be simple, lightweight, and offer flexibility. This flexibility the enFuse offers is one of the greatest advantages.
The enFuse is capable of delivering any volume from 5 to 25 mL.
The enFuse also offers flexibility for the place of delivery, whether in a hospital or clinic, in CVS or Walgreens or at home by a healthcare provider or self-administered by the user.
Enable Injections provides our Pharma partners the ultimate flexibility in the manufacture and distribution of their drug because enFuse utilizes the original container closure. The container, the manufacturing process, storage or the distribution of the drug does not need to be modified in order to utilize the enFuse.
enFuse can be paired with either a vial transfer system or a syringe transfer system for transfer of drug from the original container into the enFuse at the time of use.
The vial transfer system transfers the entire contents of the vial automatically into the injector. The syringe transfer system is ideal for weight based dosing or variable dosing and is ideal for clinical studies.
To learn more about the enFuse wearable drug delivery solution, contact us.
The investigational enFuse® system has not been approved for use by any regulatory agency and is currently not approved for commercial use.
Mehul Desai Joins Enable Injections as VP, Medical Affairs
Mehul Desai Joins Enable Injections as VP, Medical Affairs
We are pleased to announce Mehul Desai, PharmD, MBA, has joined Enable Injections as Vice President of Medical / Clinical Affairs. Desai will lead Enable’s enFuse® medical and clinical programming to support publication, new partnerships, and regulatory approvals.

Desai brings an array of experience in medical and clinical affairs across the pharmaceutical space. He most recently served as Associate Medical Director for argenx, driving the strategic direction, supporting clinical trial execution, and contributing to business development activities across various therapeutic areas. Prior to argenx, his experience includes field medical assignments in rare disease, nephrology and neurology for Mallinckrodt Pharmaceuticals, nephrology and hematology for Alexion Pharmaceuticals, and post-doctoral fellowship work in Medical Affairs for Johnson & Johnson. Desai also served as a clinical instructor of Pharmacy Practice and Pharmacy Administration at the University of the Sciences in Philadelphia.
“I am honored to be joining Enable Injections as the Vice President of Medical / Clinical Affairs and look forward to progressing the breadth and depth of the company’s partnerships, with the goal of getting safe and effective therapies to patients while demonstrating mutual benefit to all stakeholders,” said Dr Desai. “The enFuse® platform has the potential to disrupt the drug delivery space and I am excited to continue the momentum.”
Desai holds a bachelor’s degree in Biochemistry and Business Foundations from Indiana University, Bloomington; a Doctor of Pharmacy from Purdue University; a post-doctoral fellowship with Johnson & Johnson Consumer, Inc.; and a Master of Business Administration in Pharmaceutical and Healthcare Business from the University of the Sciences in Philadelphia.
“We are very excited to welcome Mehul to the Enable team. With his valuable experience in medical and clinical affairs in various roles in the pharma industry, we look forward to the positive impact he will have on our team and collaboration with our pharma partners,” said Mike Hooven, President and CEO, Enable Injections.
To learn more about the enFuse, contact us.
Enable Injections Enhances Board with Addition of John DeFord, Ph.D.
New director, John DeFord, Ph.D., adds global medical technology experience to Enable Injections, strengthening its mission to lead drug delivery innovation worldwide.
CINCINNATI, August 11, 2022 /PRNewswire/ — Enable Injections, Inc. (“Enable”), a company developing and manufacturing the enFuse® platform of investigational wearable drug delivery systems, is pleased to announce the appointment of John DeFord, Ph.D. to the company’s Board of Directors, effective July 29, 2022.
Enable Injections’ enFuse is an innovative drug delivery technology designed to subcutaneously (SC) deliver large volumes of up to 50mL for a wide range of therapies and diseases, dedicated to improving the patient experience.
 Dr. DeFord brings over three decades of expertise as a global medical technology executive, most recently the executive vice president and chief technology officer for Becton, Dickinson and Company (BD) (BDX), a global medical technology company. Prior to BD, Dr. DeFord served in senior science, technology and leadership roles of increasing responsibility at organizations such as C.R. Bard and Cook Incorporated, with additional experience in venture capital as managing director of Early Stage Partners venture capital fund. He is currently also a member of the board of directors at Nordson Corporation (NDSN), NuVasive (NUVA), and Blue Spark, Inc. DeFord graduated from Purdue University with a bachelor’s degree and master’s degree in electrical engineering, and a Ph.D. in electrical/biomedical engineering, dedicating his career to innovative medical technologies to save lives, enhance quality of life and improve the patient experience.
Dr. DeFord brings over three decades of expertise as a global medical technology executive, most recently the executive vice president and chief technology officer for Becton, Dickinson and Company (BD) (BDX), a global medical technology company. Prior to BD, Dr. DeFord served in senior science, technology and leadership roles of increasing responsibility at organizations such as C.R. Bard and Cook Incorporated, with additional experience in venture capital as managing director of Early Stage Partners venture capital fund. He is currently also a member of the board of directors at Nordson Corporation (NDSN), NuVasive (NUVA), and Blue Spark, Inc. DeFord graduated from Purdue University with a bachelor’s degree and master’s degree in electrical engineering, and a Ph.D. in electrical/biomedical engineering, dedicating his career to innovative medical technologies to save lives, enhance quality of life and improve the patient experience.
“Enable Injections is at the forefront of both simplifying the therapeutic delivery of critical medications and enhancing quality of life for the patient,” said Dr. DeFord. “I am excited to join the Board of Directors at this important time in the company’s growth and development; and I look forward to working with the management team and Board to help accelerate translation of this important technology to the clinical setting.”
“We are pleased to welcome Dr. DeFord as a new director to Enable’s Board,” said Mike Hooven, President and CEO of Enable Injections. “We believe adding depth of experience in the global medical technology space such as Dr. DeFord’s is key to the strong growth of our business.”
View original content to download multimedia: https://www.prnewswire.com/news-releases/enable-injections-enhances-board-with-addition-of-john-deford-phd-301604379.html
Enable Injections Enhances Board with Addition of Three New Directors
New directors add meaningful operating, investing and delivery systems experience to Enable Injections, strengthening its mission to lead drug delivery innovation worldwide.
CINCINNATI, OH—June 02, 2022 /PRNewswire/ – Enable Injections, Inc. (“Enable”), a company developing and manufacturing the enFuse® platform of investigational wearable drug delivery systems, is pleased to announce the appointment of James (“Jim”) Collins, Rebecca (“Beckie”) Robertson, and Alec White to the company’s Board of Directors, effective May 31, 2022. In connection with these appointments, Enable increased the size of its Board to nine members.
Jim Collins brings over two decades of expertise as a global biopharma executive, most recently at Sanofi and Eli Lilly, in device research and development, design, packaging and delivery. Jim dedicated his career to developing and launching multiple innovative drug delivery technologies to improve patient convenience. These included the market-leading insulin KwikPen Platform, the Trulicity Pen Platform, the Talz Pen Platform, the Savvio Pen, the Forteo Pen, and a number of other first-to-market delivery systems.
Beckie Robertson is Principal of Longridge Business Advisors and co-founder of Versant Ventures, a leading healthcare venture capital firm with $3.2 billion under management. Beckie, who specializes in early-stage medical devices and diagnostics, has over 30 years of executive experience in the medical device industry. Beckie’s career encompasses venture capital and operating experience in medical products as an engineer, entrepreneur, corporate executive, and investor.
Alec White is Senior Analyst at Magnetar Capital, a leading alternative investment firm with over $13 billion under management. Alec covers pharmaceutical and therapeutic investments and has over a decade of buy side investment experience. Alec and the Magnetar team provide extensive contacts across the financial and biotech communities and deep healthcare investing expertise.
 “We are pleased to welcome Jim, Beckie, and Alec as new directors to Enable’s Board,” said Mike Hooven, President and CEO of Enable Injections. “Their collective experience reflects a diversity of perspectives, skills, and backgrounds, which we believe is key to effectively implementing strong governance to help manage and facilitate growth of our business. We look forward to benefiting from the new directors’ guidance as we continue to execute on our strategy and seek to enhance value in the drug delivery space.”
“We are pleased to welcome Jim, Beckie, and Alec as new directors to Enable’s Board,” said Mike Hooven, President and CEO of Enable Injections. “Their collective experience reflects a diversity of perspectives, skills, and backgrounds, which we believe is key to effectively implementing strong governance to help manage and facilitate growth of our business. We look forward to benefiting from the new directors’ guidance as we continue to execute on our strategy and seek to enhance value in the drug delivery space.”
About Enable Injections
Cincinnati-based Enable Injections is a global healthcare innovation company developing and manufacturing drug delivery systems designed to improve the patient experience. Enable’s body-worn enFuse® delivers high-volume pharmaceutical and biologic therapeutics via subcutaneous administration, with the aim of improving convenience, supporting superior outcomes, and advancing healthcare system economics. For more information, please visit www.enableinjections.com.
View original content to download multimedia: https://www.prnewswire.com/news-releases/enable-injections-enhances-board-with-addition-of-three-new-directors-301560279.html
Large-Volume Wearable Drug Delivery Technology Is Clinically Proven
Opening Summary
Does large-volume wearable drug delivery technology really work? And is it clinically proven? Experts addressed these questions and more in a recent webinar. Read on to learn more and access the full webinar discussion below. Re-cap:
- Emerging wearable technology enables flexibility of delivery volumes from 5 to 50 mL
- Large-volume SC delivery technology brings care closer to the patient
- Convenience in care is important to patients
Does large-volume wearable technology for subcutaneous (SC) delivery work? Yes. Emerging innovative technology enables large-volume delivery and this innovation is clinically proven to work.

Mike Hooven, President and CEO, Enable Injections
Mike Hooven: Yes, but current technologies delivering volumes greater than 10 mL are complicated, bulky, and non-wearable.
As a delivery technology innovator, Enable Injections recognized the only way to deliver high volumes in a wearable technology is to design with simplicity. For the enFuse, a silicone tube acts as both the drug container and the power supply. This design enables the most efficient size for its delivery volume, allowing flexibility of a range from 5 to 50 mL.
enFuse’s unique elastomeric technology delivers therapeutic at a low pressure. From early clinical data, we have relative confidence that this low-pressure injection, which is responsive to the pressure in the subcutaneous tissue, translates to resulting clinical benefits. We’ve built and tested more than 100,000 enFuse units, executed 4 clinical studies, and have several upcoming studies. Our preliminary data show:
- Overwhelming patient preference
- A high degree of comfort
- Reduced adverse events
- Improved dose accuracy

Dr. Len Lichtenfeld, Chief Medical Officer, Jasper Health
Why would a physician benefit from large-volume subcutaneous delivery technology? It allows you to start taking the care closer to the patient.
Dr. Len Lichtenfeld: There’s no reason you can’t have a more efficient effective system within a clinic. This allows you to start taking the care closer to the patient. It benefits the clinician, reduces the stress on the system, and opens up options for the people who are coordinating the care, the people who are paying for the care, and most importantly, the people who are receiving the care.
What criteria should I have when selecting technology? Select a delivery system that has 1) a high level of confidence, 2) is simple, and 3) utilizes a good plan.

Jim Collins, Drug Delivery Device Expert
Jim Collins: Why should pharma companies be interested and how do they go about making these decisions? There are three key points:
- High level of confidence. It’s very important to select delivery systems that have a high probability of success with the molecule.
- Simplicity always wins. You want to pick the system that is simplest to use.
- A good plan is essential. A molecule paired with a new delivery technology has a higher chance for success with a good plan in place.
Why would a large-volume SC delivery technology be beneficial for the patient? The convenience aspect, or being able to provide an overall time savings to the patient, is most important.

Elizabeth Shaheen, Biopharma Strategist
Elizabeth Shaheen: In a 488-patient preference study which looked at SC trastuzumab, 90% of patients preferred SC administration over IV. 80% explained time savings was the greatest benefit. If you look at the prior HannaH study with SC trastuzumab, the duration of subcutaneous injections was only one to five minutes versus 30 to 90 minutes for the patient to receive an IV. You see an even greater benefit where a 3,4,7-hour infusion is minimized to a 5-minute injection. That patient is also having to travel to the IV clinic, so time-savings extends to the commute for patients and caregivers.
Closing Summary – Webinar Access
enFuse technology is not only simple, but also feasible.
Read more from our first in this series. Access the full webinar content here: At-Home Drug Delivery of Large Molecule Medicines. To learn more about partnering with Enable Injections and our innovative enFuse®, contact us.
Large-Volume Subcutaneous Delivery Is Feasible
Current large-volume drug delivery methods generally require the patient and provider to be in the clinic. However, the desire to treat patients outside the clinic is growing, not only in the era of COVID-19, but also as treatment durations grow and disease is caught earlier. In our latest webinar, Pharma leaders, a KOL, and an innovator discussed technologies that can aid at-home patient-administered drug delivery – and how improving patient convenience can result in product differentiation and revenue growth. This post highlights the discussion around the first key point of the webinar: “Large-volume subcutaneous (SC) delivery is feasible”.

Jim Collins, Drug Delivery Device Expert
Is large-volume SC delivery feasible? Based on all the data we are seeing, yes.
Jim Collins: Based upon all the data we’re seeing, it [large-volume SC delivery] clearly is. Several studies done by different pharma companies began to demonstrate that delivery of 2, 3 mL was feasible. The Repatha® device demonstrated 3 mL delivery and then subsequent studies have shown the delivery of 8, 14 mL subcutaneously without enhancers is feasible.
Does it make sense from the pharma perspective? Yes, from a clinical and convenience advantage perspective.
Jim Collins: Pharma’s interest is based upon clinical advantages or convenience advantages to the patient and the health care professional. First, clinically there is a reduction in adverse events with SC delivery versus IV. Second, there’s a lot of data in drug delivery that tells us that more convenient delivery is going to provide a key differentiation moving forward.

Elizabeth Shaheen, Biopharma Strategist
What benefits would large-volume SC delivery provide for the patient? Convenience, ease of administration, and a lessened financial burden.
Elizabeth Shaheen: I want to humbly admit I’m not able to speak on behalf of all patients because clearly patient preference is unique to each individual patient. Based on patient reported outcome studies that compare SC delivery with IV infusion – findings center around three key benefits of SC delivery:
-
-
-
-
- Convenience
- Ease of administration
- Lessened financial burden
-
-
-
Does large-volume SC delivery make sense from the provider and patient perspective? Yes, this makes a lot of sense and allows us to move cancer care forward.

Dr. Len Lichtenfeld, Chief Medical Officer, Jasper Health
Dr. Len Lichtenfeld: Oncology care really has to be a team effort. We have the phenomena occurring called “value-based care” – or providing the best care to the most people at the most reasonable costs with safety and convenience in mind. Ultimately, a distinguishing factor is going to be ease of administration. From the pharma perspective, there are the technology pieces that Enable has been working on diligently to make care more convenient. From the clinician level, as well as the patient and the caregiver level, this makes a lot of sense and really allows us to move cancer care forward in the next century.
What characteristics of large-volume SC delivery are important? Comfort, convenience, and discreet.

Mike Hooven, President and CEO, Enable Injections
Mike Hooven: Based on Enable’s 10 years of experience in developing the unique enFuse® product, I do believe that the patients and providers will drive the acceptance and growth of our technology. We’ve done over 100 generative and formative Human Factors studies with virtually every patient demographic, and we found three clear and common characteristics expressed – about the enFuse technology design – by patients and providers:
-
-
- Comfort
- Convenience
- Discreet
-
Access the full webinar content here: At-Home Drug Delivery of Large Molecule Medicines. To learn more about partnering with Enable Injections and our innovative enFuse® drug delivery technology, contact us.
At-Home Drug Delivery of Large Molecule Medicines – The Future Is Now
At-Home Drug Delivery of Large Molecule Medicines
Current large volume drug delivery methods generally require the patient and provider to be in the clinic. However, the desire to treat patients outside the clinic is growing, not only in the era of COVID-19, but also as treatment durations grow and disease is caught earlier. For drug developers invested in early and chronic disease areas such as oncology, respiratory disease, or neuroscience, convenient drug delivery is a brand necessity. Dosing outside the clinic has traditionally been challenging. But new delivery technologies are facilitating this possibility, leading to improved patient compliance and better outcomes.
In our latest webinar, Pharma leaders, a KOL, and an innovator discuss technologies that can aid at-home patient-administered drug delivery – and how improving patient convenience can result in product differentiation and revenue growth.
The webinar covered three key points on subcutaneous drug delivery – including links to the blog recap series:
- Large-Volume Subcutaneous Delivery Is Feasible (link).
- Benefits of Large-Volume Wearable Drug Delivery Technology (link).
- When should Pharma engage with Large-Volume Subcutaneous Delivery Technology?
Watch the webinar recap video below for glimpse into the panel discussion.
Access the full webinar content here: At-Home Drug Delivery of Large Molecule Medicines.
To learn more about partnering with Enable Injections and our innovative enFuse® drug delivery technology, contact us.
Large-Volume Subcutaneous Delivery
Large-Volume Subcutaneous Delivery
Clinical summary on the accuracy, safety, and efficacy of large-volume subcutaneous delivery
Why IV to SC?
IV Facts
- More than 1 billion IVs are placed globally each year1.
- Peripheral IV cannulation difficulty occurs in up to 24% of adult patients and 37% of pediatrics requiring a cannula.2
SC Advantages

Is SC Delivery Safe and Effective?
A 2019 study12 concludes the following pharmacokinetic and safety data comparing IV vs. SC administration:
Safety:
“Treatment emergent AEs across all cycles occurred in 24 (96%) patients treated with PF-06801591 IV and in 15 (100%) treated with SC administration of the drug. Grade 3 or higher AEs occurred in 11 (44%) and 6 (40%) patients treated with PF-06801591 IV and SC, respectively.”12
Efficacy:
![]()
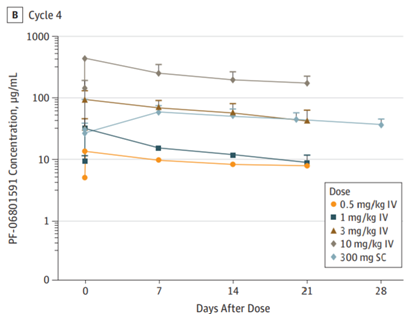
“The safety and tolerability [of IV and SC] …appear to be comparable. Exposure following SC administration was within the expected efficacious dose range.” 12
How Does enFuse® Work?
enFuse technology – simplified
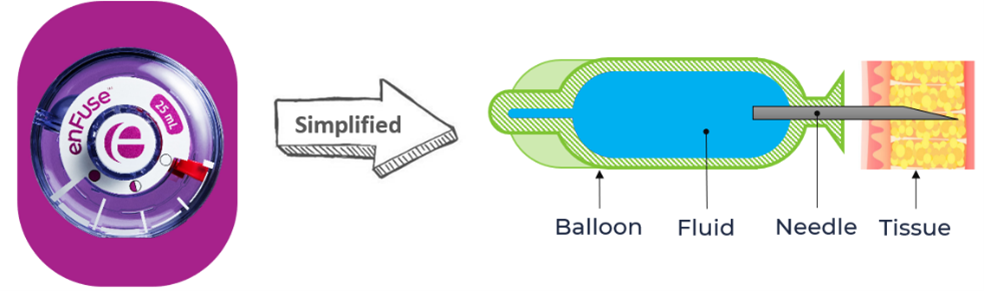
Clinical Summary
- 4 Clinical Trials
- 3 completed and 1 ongoing
- 6 Products and 3 Pharma Partners
- >600 Devices tested in clinical studies
- Up to 3 devices worn simultaneously and delivered volume ranges 1.5 to 50mL
Outcomes
- Bioequivalence met (all studies) 13 – No safety issues
- 30% reduction in Adverse Events vs. standard of care13
- No injection site reactions13
- Improved Dose Accuracy, reduced Site Leakage13
Enable Injections, Inc.
Redefining drug delivery for the benefit of patients, providers, and partners.
Enable Injections is a clinical-stage company developing and manufacturing on-body delivery systems designed to improve the patient drug delivery experience. Enable’s enFuse® system subcutaneously delivers large volumes of up to 50 mL of high-viscosity therapeutics. Contact us to further discuss large volume subcutaneous delivery of large molecule medicines.
To learn more about Enable’s Manufacturing, click here.
Clinical summary on the accuracy, safety, and efficacy of large-volume subcutaneous delivery
Sources:
- Beecham, G, et al,. “Peripheral Line Placement.” StatPearls; 2021. (link)
- Rodriguez-Calero, M., et al. “Defining risk factors associated with difficult peripheral venous cannulation: a systematic review and meta-analysis.” Heart & Lung, Vol 49, issue 3, May 2020, pp 273-286. (link)
- Subcutaneous monoclonal antibody in cancer care: cost-consequence analysis of subcutaneous rituximab in patients with follicular lymphoma 5. De Cock, E; et al. (link)
- Switching from IV to SC IL-6 Receptor Inhibitor Therapy Shows No New Safety Signals, Jan 2021, (link)
- Update on the subcutaneous administration of rituximab in Canadian cancer centers (link)
- The optimal choice of medication administration route regarding intravenous, intramuscular, and subcutaneous injection, (link)
- Switching from IV to SC Rituximab Saves Staff Time and Money (link)
- FDA H.R.3590 Patient Protection and Affordable Care Act (link)
- Efficacy of SC formulations: Comparison of SC versus IV administration of rituximab as maintenance treatment for follicular lymphoma: results from a 2-stage, phase IB study (link)
- Payer Perspectives on Intravenous versus Subcutaneous Administration of Drugs 2021 (link)
- Health Economics study, 2019. Ref. Enable Injections.
- Johnson, M., et al. “Assessment of SC vs IV Administration of Anti-PD-1 Antibody PF-06801591 in Patients with Advanced Solid Tumors, a Ph 1 Dose Escalation Trial.” JAMA Oncology, 30MAY2019. (link)
- Data on file, Enable Injections, Inc.
Sanofi announces partnership with Enable Injections, Inc. on subcutaneous delivery for Sarclisa®
In a press release today, Sanofi announces partnership with Enable Injections, Inc. on subcutaneous delivery for Sarclisa®.
Sanofi announces €300 million collaboration with Blackstone Life Sciences to advance an innovative treatment for multiple myeloma
Enable Injections is delighted with the public news, announcing our partnership with Sanofi for the subcutaneous formulation and delivery of the anti-CD38 antibody Sarclisa®, to treat patients with multiple myeloma (MM).
“For the Sarclisa® subcutaneous formulation delivery, Sanofi has partnered with drug delivery technology innovator company Enable Injections, Inc. to advance the development of a subcutaneous delivery for Sarclisa® with the goal of offering a unique patient-centric treatment experience.” Click to read the full press release.

To learn more about partnering with Enable Injections and our innovative enFuse® drug delivery technology, contact us.
Reimagining Patient Access to Treatment
Reimagining Patient Access to Treatment
by Mike Hooven, CEO, Enable Injections – LinkedIn Published
Just imagine. Your doctor tells you that your health issues are treatable. Good news, right?
But then, she explains, the treatments involve at least 3 hours each week to receive the medicine. Not such good news.
If you are a student or employee, parent or child, 3 hours per week is disruptive. Those hours add up, and when factoring in travel and preparations, it becomes a significant part of your life. Some would call this a part-time job. Is it really that surprising that the patient adherence rate is less than 50%1?
There has to be a better way.
In my first article of this series, I wrote about the difficulties cancer patients face in getting the care they need, especially during the pandemic. In my second, I discussed the need for innovation to improve patient access to care. Today, I’m calling on more compassion and appreciation of the patient’s burden.
IV treatments are lengthy, costly, difficult to give and receive, and require healthcare teams for administration at home or in the clinic. Autoinjectors and syringes are quick, but have strict delivery volume limitations which necessitate multiple injections at once, often creating confusion and a real aversion for patients opposed to needles.
We must improve the patient experience to help patients get the treatments they need. We must find alternatives to bothersome sit-down infusions, or multiple sticks with large needles.
Solutions do exist, and are worthwhile. The right subcutaneous (SC) delivery solution has the potential to be transformative for both patients and the healthcare system, freeing up patient time, improving patient experience, and providing flexibility for healthcare resources. 
But what are the drawbacks, and are they really legitimate? Some common misconceptions about SC administration include:
1. Dose: Can large volumes of therapeutic be delivered via SC?
Yes. Wearable delivery systems allow patients to go about their lives while they are receiving their therapeutic. And the right large volume wearable delivery system never exposes the needle to the patient.
2. SC capability: Can the skin really absorb medication in large volumes?
Yes. Research has been done to prove safety and efficacy of large volumes of therapeutics when administered via SC. In addition, large volumes of therapeutic do not need additional delivery enhancement to be effective.2
3. Ease: Is administering medication through the skin (SC) really an improvement?
Yes, the impact of SC dosing has been clinically proven to reduce overall time for both patient and provider. When home administration occurred in clinical studies, SC resulted in improved patient quality of life.3, 4 SC delivery solutions, specifically large volume wearable injectors, will allow patients to access improved methods of self-administration over current cumbersome SC systems.
The good news for patients, healthcare providers, pharma, and payers is that there is already technology that delivers the therapeutics that patients need – with large volume wearable injectors in particular providing flexibility for the patient and provider.
With the right subcutaneous delivery system, that 3 hours per week at an infusion clinic or hospital could be significantly reduced or transferred completely to the home. At Enable, we believe the right therapeutic can help patients to get their health back, but the right delivery system can help patients to get their lives back.
Learn More:
Join us in discussion on this topic at upcoming industry webinar with key opinion leaders on April 28 at 10am Eastern US. Click here to register.
References:
1) Sabaté E, ed. Adherence to Long-Term Therapies: Evidence for Action. Geneva, Switzerland: World Health Organization; 2003. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3068890/
2) Johnson ML, Braiteh F, Grilley-Olson JE, et al. Assessment of Subcutaneous vs Intravenous Administration of Anti–PD-1 Antibody PF-06801591 in Patients With Advanced Solid Tumors A Phase 1 Dose-Escalation Trial. JAMA Oncology. July 2019; 5(7): 999-1007. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6547134/
3) Abolhassani H, Sadaghiani MS, Aghamohammadi A, Ochs HD, Rezaei N. Home-based subcutaneous immunoglobulin versus hospital-based intravenous immunoglobulin in treatment of primary antibody deficiencies: systematic review and meta analysis. J Clin Immunol. 2012;32:1180–1192. doi: 10.1007/s10875-012-9720-1.
4) Bittner B, Richter W, Schmidt J. Subcutaneous Administration of Biotherapeutics: An Overview of Current Challenges and Opportunities. BioDrugs. 2018;32(5): 425-440. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6182494/
